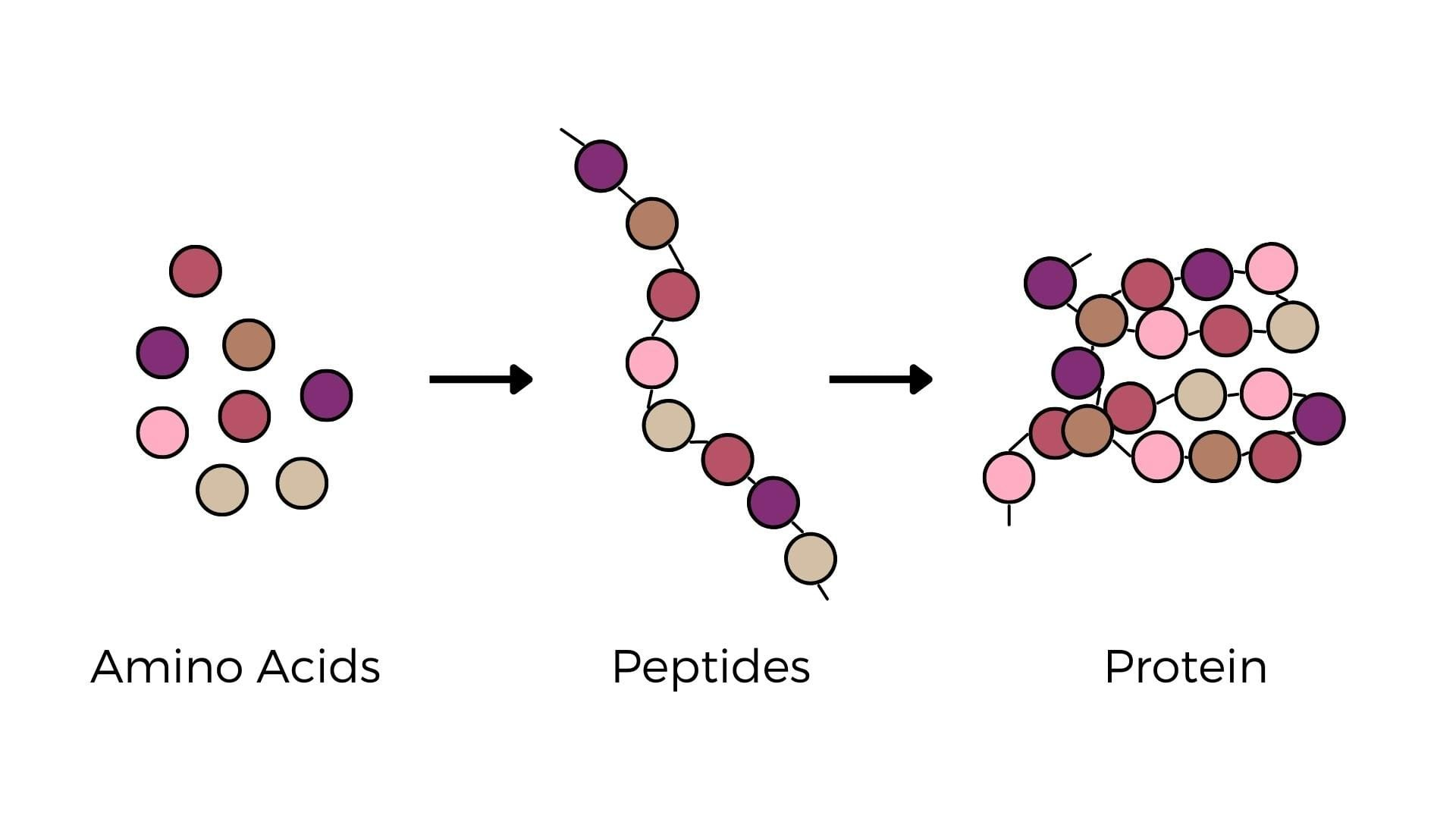
Peptide
Welcome


Peptide Profile
Database number
prediction Tools
Anticancer peptides (ACPs) are small molecules composed of amino acids that exhibit inhibitory or cytotoxic effects on cancer cells. They are typically derived from natural proteins or designed through artificial synthesis, featuring unique structural characteristics and functions. ACPs exert their effects through various mechanisms, including membrane disruption, induction of apoptosis, inhibition of the cell cycle, and modulation of the immune system.Due to their small molecular weight, high stability, and strong targeting capabilities, ACPs hold significant potential in cancer treatment and are an important focus of research.
Reference: Norouzi, Norouzi P, Mirmohammadi M, Tehrani M H H. Anticancer peptides mechanisms, simple and complex[J]. Chemico-Biological Interactions, 2022, 368: 110194.
Antimicrobial peptides (AMPs) are short peptide molecules with antimicrobial activity, widely distributed in humans, animals, plants, and microorganisms. They exert their effects by interacting with microbial cell membranes, disrupting membrane integrity and causing cell death, or by inhibiting cell wall synthesis and interfering with internal molecular targets. AMPs exhibit broad-spectrum activity against various microorganisms, including bacteria, fungi, viruses, and certain parasites. Compared to traditional antibiotics, AMPs have a lower tendency to induce resistance, and they offer strong selectivity with low toxicity. Due to these advantages, AMPs are considered promising candidates for the development of new antimicrobial drugs and have extensive application potential in clinical medicine, health products, and cosmetics.
Reference:Bahar A A, Ren D. Antimicrobial peptides[J]. Pharmaceuticals, 2013, 6(12): 1543-1575.
Neuropeptides (NPs) are short chains of amino acids secreted by the nervous system that act as modulators in neurocommunication. Typically consisting of several dozen amino acids, neuropeptides play a crucial role in regulating a variety of physiological processes, including mood, pain perception, appetite, sleep, learning, memory, and stress responses. By binding to specific receptors, neuropeptides influence the communication between neurons and help regulate various bodily functions. There are many types of neuropeptides, each with highly specific functions. Common neuropeptides include endorphins, vasopressin, calcitonin gene-related peptide (CGRP), and prolactin-releasing factors, among others. These neuropeptides exert their effects either through local release within the nervous system or by influencing specific brain regions, serving as chemical signals between cells. Neuropeptides have significant potential clinical applications, particularly in mood regulation, pain relief, and the treatment of neurodegenerative diseases. As neuroscience continues to evolve, neuropeptides are increasingly seen as a valuable resource for drug development and therapeutic interventions.
Reference:Van Den Pol A N. Neuropeptide transmission in brain circuits[J]. Neuron, 2012, 76(1): 98-115.
Anti-tuberculosis peptides (ATPs) are a class of short peptides with anti-tuberculosis activity that can effectively inhibit or kill Mycobacterium tuberculosis. These peptides are typically derived from the natural immune system, microbial metabolites, or through rational design and optimization. ATPs exhibit unique mechanisms of action, such as penetrating the bacterial cell wall, disrupting membrane stability, or inhibiting key enzyme activities, thereby suppressing the growth of tuberculosis bacteria. Due to their lower risk of inducing resistance and strong target specificity, ATPs offer a promising solution for the treatment of multidrug-resistant (MDR-TB) and extensively drug-resistant tuberculosis (XDR-TB). Studies suggest that optimizing amino acid sequences, improving peptide stability, and enhancing intracellular delivery efficiency may further advance ATPs as potential therapeutic candidates for tuberculosis treatment.
Reference: Zhang, Shengping, Iman Kavianinia, and Margaret A. Brimble. "Naturally occurring antitubercular cyclic peptides." Tetrahedron Letters 60.50 (2019): 151339.
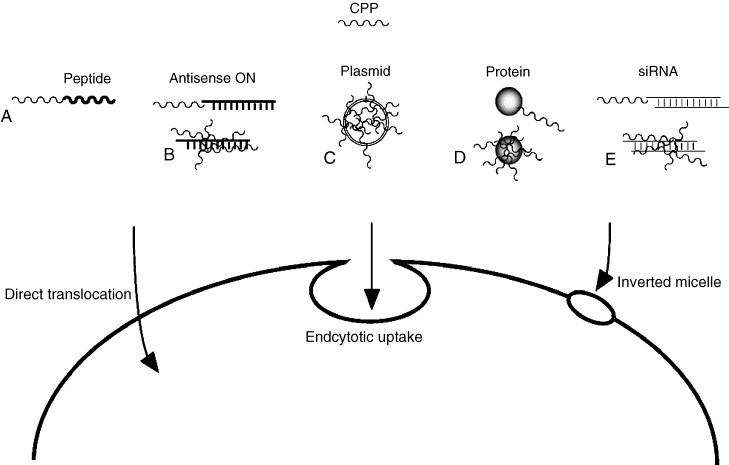
Cell Penetrating Peptides (CPPs) are short peptides that have the ability to penetrate biological membranes and enter cells. They are typically composed of 10 to 30 amino acid residues and possess characteristics such as positive charge or hydrophilicity. CPPs can enter cells either by directly crossing the cell membrane, via endocytosis, or a combination of both, making them widely applicable in drug delivery, gene therapy, and molecular probes. The mechanisms of CPP action generally include two pathways: one is through non-receptor-mediated translocation (such as direct penetration), and the other is via endocytosis, followed by intracellular delivery. Research has shown that the interaction between CPPs and cell membranes usually relies on the positive charge of the peptides and the hydrophobicity of the amino acids. Common CPPs include TAT peptide (derived from the HIV transactivator of transcription), Penetratin (derived from amphibian antimicrobial peptides), and Polyarginine (a poly-arginine peptide). These peptides can effectively deliver small molecule drugs, nucleic acids, proteins, and other large molecules into cells, and are also used as tools for delivery systems in research. However, while CPPs hold significant potential in drug delivery systems, challenges such as biodegradability and cytotoxicity remain issues that need to be addressed.
Reference:Järver P, Langel Ü. Cell-penetrating peptides—a brief introduction[J]. Biochimica et Biophysica Acta (BBA)-Biomembranes, 2006, 1758(3): 260-263.
© BioAi-Lab All Rights Reserved